Bangladesh’s First Covid Vaccine Receives Nod For Human Trials
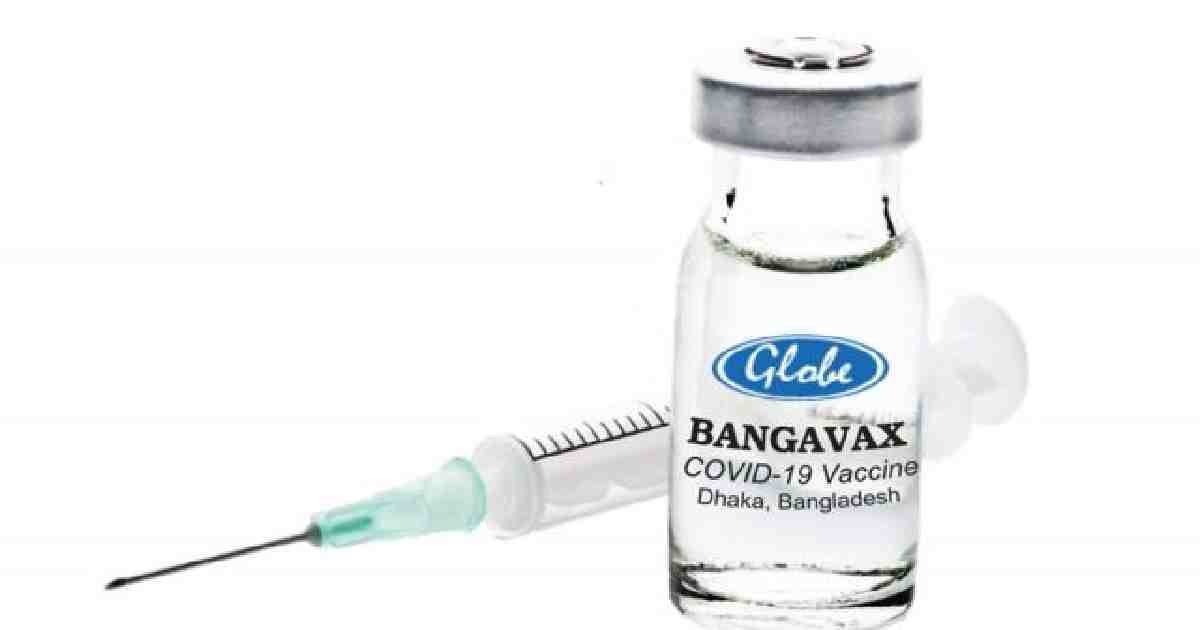
Bangladesh’s first homegrown vaccine has received approval from the Bangladesh Medical Research Council (BMRC) for human trials.
Senior manager for quality and regulation AT Directorate General of Drug Administration (DGDA) stated that the manufacturer of Bangavax, Globe Biotech is set to send DGDA human trial protocol, reported the Daily Star.
After the single-dose vaccine, Bangavax gets assent for clinical trials from DGDA, Globe Biotech will initiate human trials, said DGDA senior manager.
Globe Biotech on October 5 the previous year, revealed that its first vaccine candidate had shown promise in pre-clinical trials on mice, reported the Daily Star.
Bangavax was initially known as Bancovid. (ANI)