The Central Government on Wednesday formed a four-member expert committee to investigate the report sent by the World Health Organisation (WHO) on the deaths of 66 children in the Gambia by consumption of cough syrups from Sonipat-based company Maiden Pharmaceuticals Limited, said official sources.
“Government is alert and formed a committee. Production of cough syrups by Maiden Pharmaceuticals Ltd has been suspended, and a lab report is awaited. Four-member expert committee has been formed to investigate the report sent by WHO,” official sources told ANI.
A committee comprising technical experts Dr. YK Gupta, Vice Chairperson, Standing National Committee on Medicines (chair); Dr. Pragya Yadav, ICMR -NIV, Pune; Dr. Arti Bahl, Division of epidemiology, NCDC, New Delhi, and AK Pradhan, JDC(I), CDSCO has been formed.
According to the reports, Atlanta-based Atlantic Pharmaceuticals Company Ltd which has permission to export medicines to the Gambia ordered combined syrups bottles which were purchased from Maiden Pharmaceuticals limited.
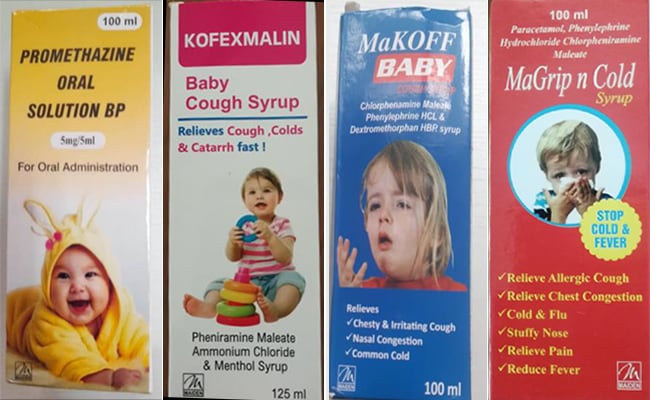
“From the preliminary inquiry of Central Drugs Standard Control Organisation (CDSCO), it has been made out that Maiden Pharmaceuticals Ltd is a manufacturer licensed by the State Drug Controller for the product’s Promethazine Oral Solution BP, Kofexnalin Baby cough syrup, MaKoff Baby Cough Syrup and MaGrip n Cold Syrup under reference, and holds manufacturing permission for these products for export only,” the sources said.
The company has manufactured and exported these products only to the Gambia.
“It is a usual practice that the importing country tests such imported medicines on quality parameters, and satisfies itself as to the quality of the products before the importing country decides to release such products for usage in the country. In the present case, it is yet not clear whether these medicines were tested in the Gambia before release,” the sources added.
The WHO has informed that as per the tentative results received by WHO, out of the 23 samples of the products under reference which was tested, four samples have been found to contain Diethylene Glycol or Ethylene glycol.
“WHO has not yet made available a certificate of analysis. It has informed that the same will be made available in near future,” the sources said.
The top officials informed that the exact “one-to-one causal relation of death” has not been provided by WHO to CDSCO although CDSCO has requested WHO twice in this regard.
Earlier in the day, the Haryana government has ordered a halt to the total production of cough syrups from Sonipat-based company Maiden Pharmaceuticals Limited.
The company has also been issued a show cause notice for 12 violations found at its manufacturing plant.
State health minister Anil Vij told ANI on Wednesday, “The samples of three drugs of the Sonipat-based pharmaceutical company, which were mentioned by WHO, have been sent to Central Drug Laboratory in Kolkata. The report is yet to come. We will take action only after the report comes. After a joint inspection by Central and State officials, 12 violations were found in the manufacturing facility. … It has been decided to shut down total production. We have sent a notice in this regard.”
The pharma company had come under scanner following the death of 66 children who had consumed the cough syrup in the Gambia.
World Health Organization (WHO) on October 5 issued a medical product alert over four India-made cough and cold syrups manufactured by Maiden Pharmaceuticals. WHO has also notified that the same cough and cold syrups could be linked to the deaths of 66 children in the Gambia. (ANI)
Read More:http://13.232.95.176/