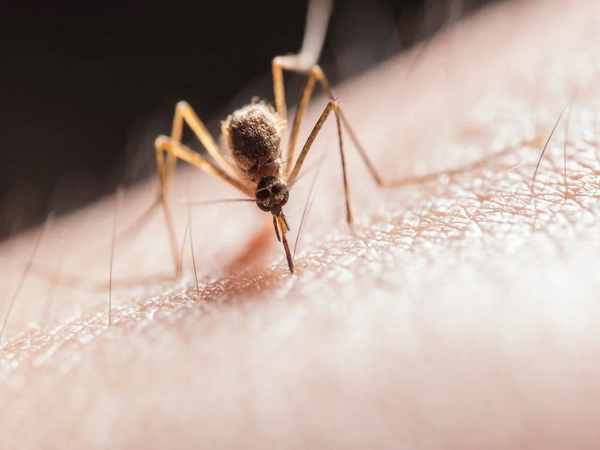
A fragrance from human skin directs mosquitoes that transmit Zika, dengue, and yellow fever toward their victims. Up until this point, the precise makeup of that smell was unknown.
The aroma that prompts a mosquito to identify and land on its victim is produced by the mixture of carbon dioxide and the compounds 2-ketoglutaric and lactic acids, according to research led by UC Riverside. This chemical concoction also promotes probing, which is the practice of piercing mouthpieces to look for blood.
This chemical concoction appears to draw female Aedes aegypti mosquitoes, carriers of the Zika, chikungunya, dengue, and yellow fever viruses, in particular. This mosquito was first discovered in Africa, but it has since spread to many tropical and subtropical countries, including the United States.
In the journal Scientific Reports, the team’s new research finding and how it was made are described in detail. “Although others have discovered substances that attract mosquitoes, many of them don’t have a noticeable, immediate impact. This one does, according to entomologist Ring Carde of UCR.
Mosquitoes employ a number of indicators, such as carbon dioxide, sight, temperature, and humidity, to find their prey. Recent studies by Carde, however, indicate that skin scents are much more crucial for identifying a bite location.
We showed that mosquitoes land on visually unclear targets that are infused with these two scents, and these targets are not connected to heat or wetness, according to Carde. Skin odour is now the main determining factor.
Given the importance of smell in mosquitoes’ ability to successfully feed on humans, Carde set out to identify the precise compounds that give humans’ fragrance such power to the insects. Lactic acid, a component of the equation, was recognised as one of the chemical components of the odour cocktail as early as 1968.
Since then, numerous studies have found that human-produced compounds, such as ammonia and carbon dioxide, also draw these insects. Carde, who has spent 26 years studying mosquitoes, concluded that these additional compounds were not potent attractants.
Carde stated, “I had a sneaking suspicion there was some unexplored aspect to the chemistry of smells tempting the yellow fever mosquito. I sought to identify the precise combination.
The 2-ketoglutaric acid could not have been identified using the methods that chemists generally employ, according to Carde. Gas chromatography would not have detected this acid, which separates substances based on their polarity and molecular weight.
Because of the intricacy of the human odour profile and the minute levels of these compounds present in sweat, said scientist Jan Bello, previously of UCR and currently with insect pest control business Provivi, “I suspect that these molecules may not have been detected earlier.”
Carde turned to Bello, who was drawing substances from the sweat on his own foot to use as a mosquito attractant. He put glass beads in his socks and wore them for four hours while walking about to gather odours.
Bello remarked that wearing the beads was similar to “squeezing stress balls full of sand, but with your feet.” They would eventually become unpleasant after doing that for a while because they would get trapped in between your toes, which is the most aggravating aspect.
The expense was worthwhile despite the hassle. Bello purified compounds from the perspiration left on the sock beads and tracked how the chemicals affected the behaviour of mosquitoes. The combo that was the most active was thus revealed.
Future research is intended to ascertain whether the same substance works against other mosquito species and why different people are prone to be bitten differently. Some are more alluring to these mosquitoes than others, but no one has yet determined why, according to Carde.
The research team is optimistic that their discovery can be used to attract, capture, and possibly kill disease-carrying mosquitoes, even though it may not provide information for the creation of new repellents.
In the end, we’re really happy that we discovered these chemicals because we weren’t always confident that we would. Thoughts don’t always pan out, but we had a suspicion they existed, Carde added. (ANI)
Read More:https://lokmarg.com/